Vertex/CRISPR to begin rolling review in US for gene-edited therapy; get FDA designations | Seeking Alpha
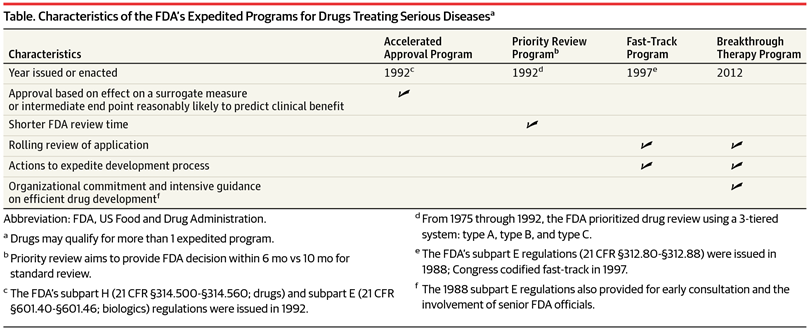
The Science Of A Biotech Valuation: How To Interpret The Value Of FDA Expedited Programs (NASDAQ:IBB) | Seeking Alpha
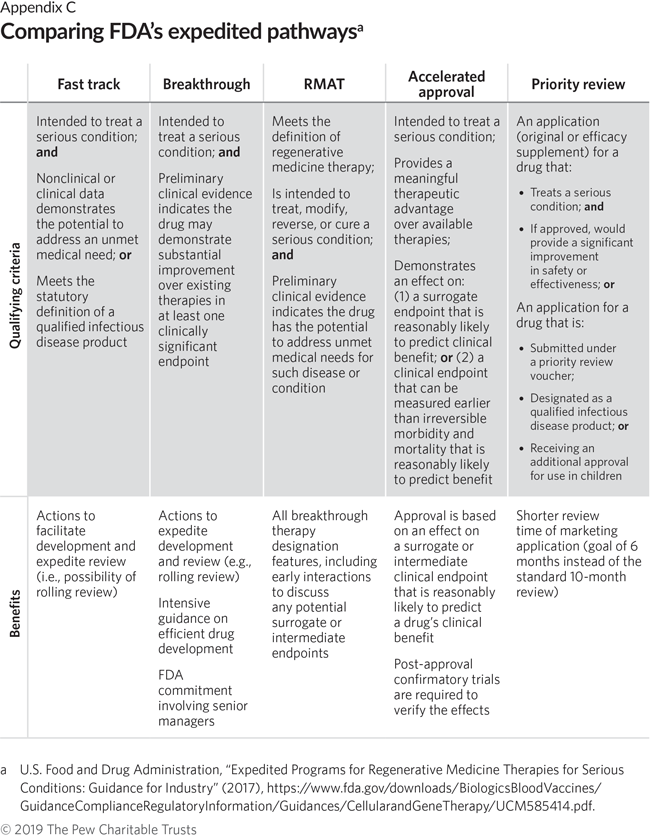
FDA's Framework for Regulating Regenerative Medicine Will Improve Oversight | The Pew Charitable Trusts

Analysis of the Real-Time Oncology Review (RTOR) Pilot Program for Approvals of New Molecular Entities | SpringerLink

CytoDyn Inc. submits the first of three main sections of its HIV Biologics License Application to FDA under rolling review

Nisarg Patel on Twitter: "Yet, we have a multitude of regulatory pathways to accelerate the development and review of COVID19 therapeutics without compromising clinical evidence. Here, we adopt elements of the Real-Time
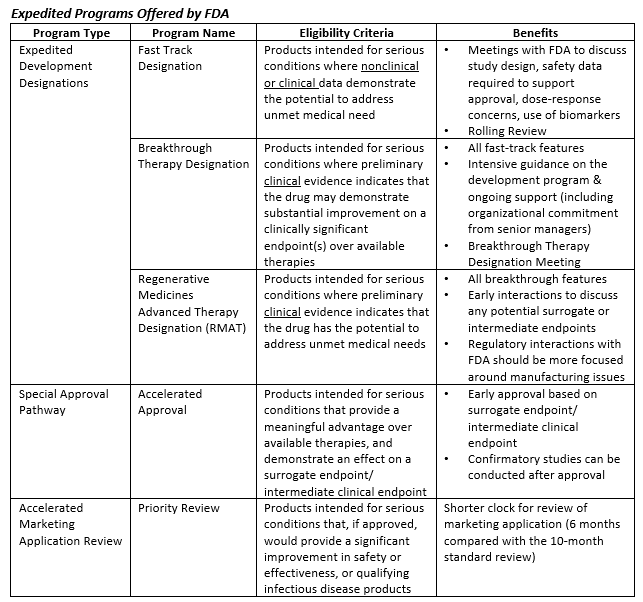
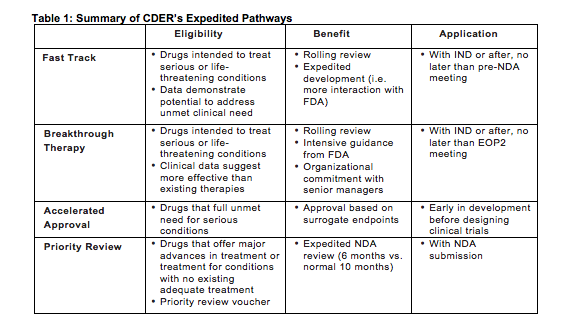
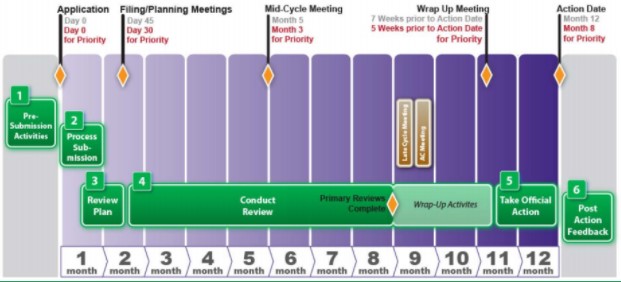
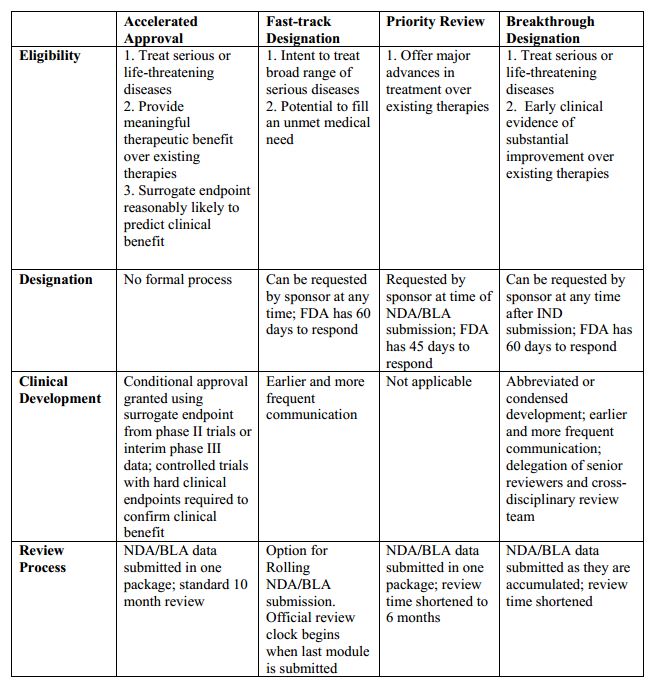
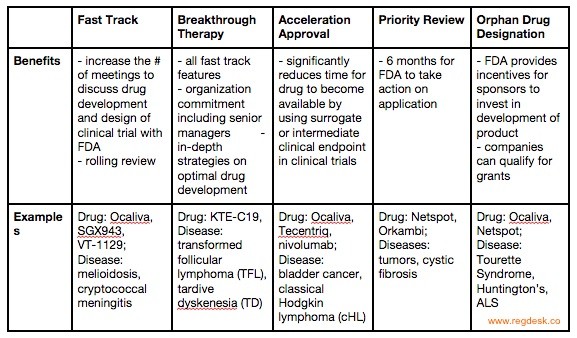
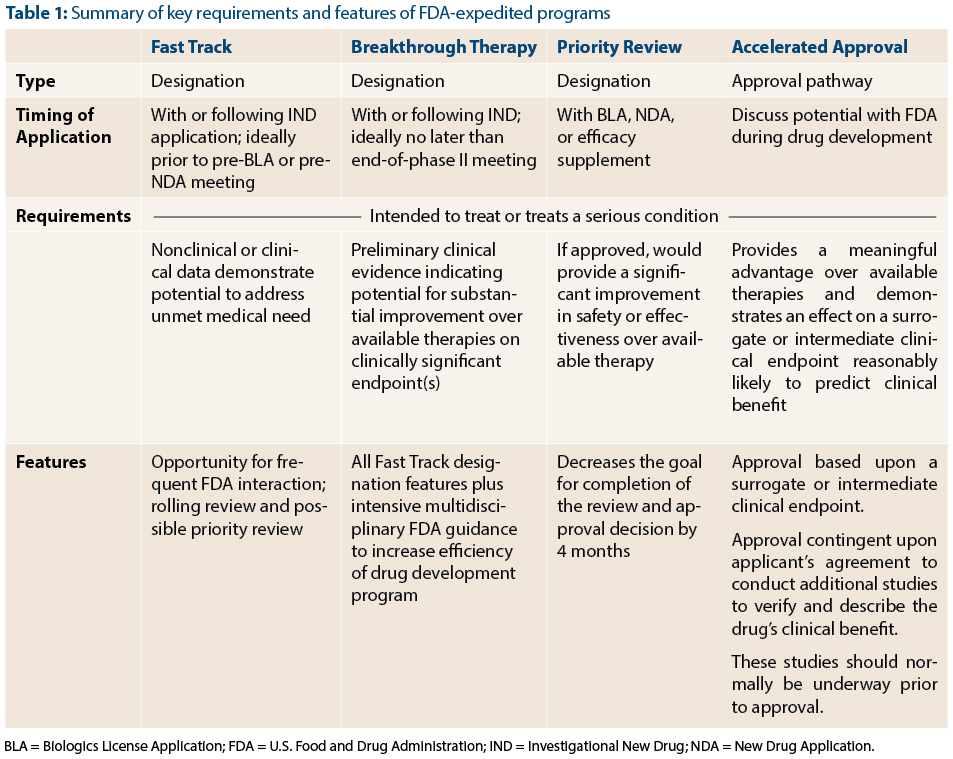
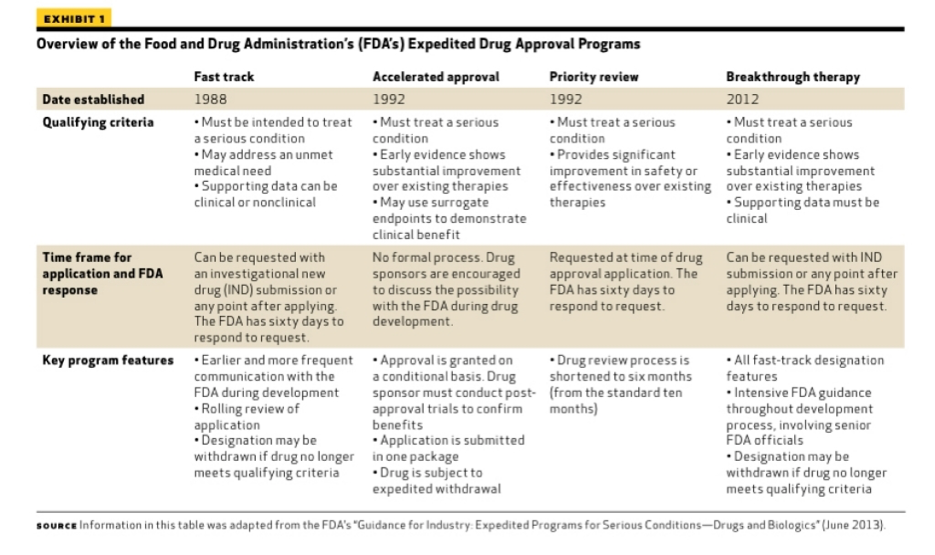
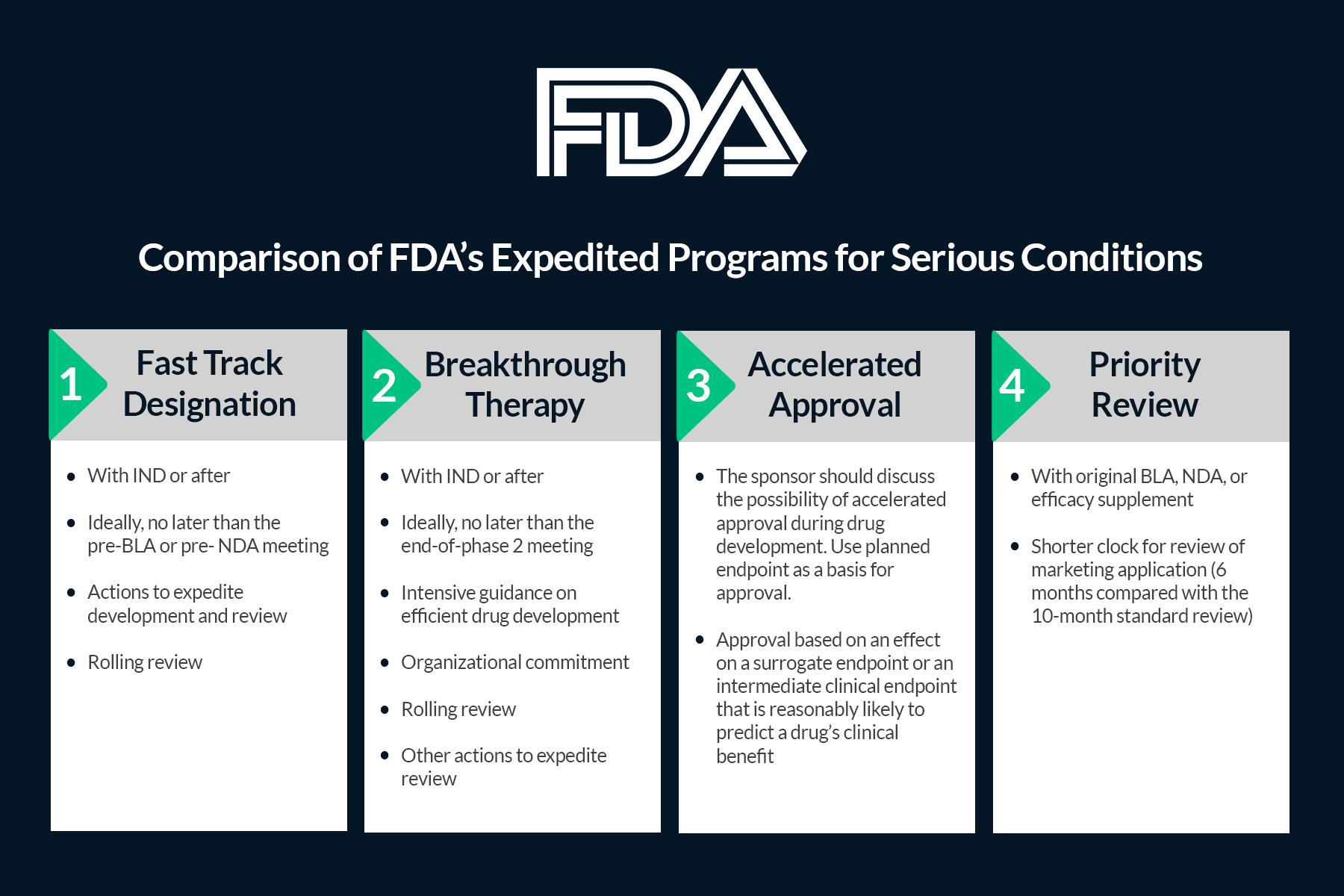
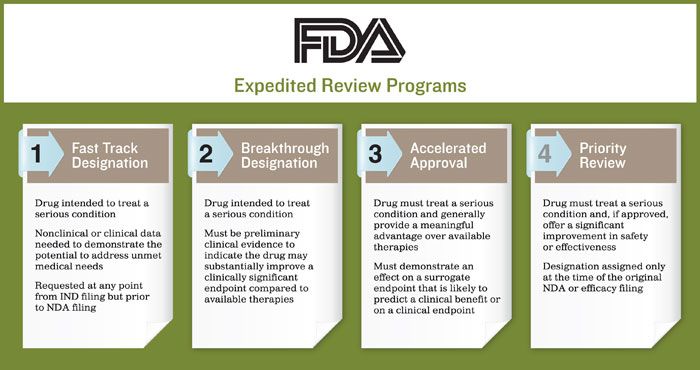
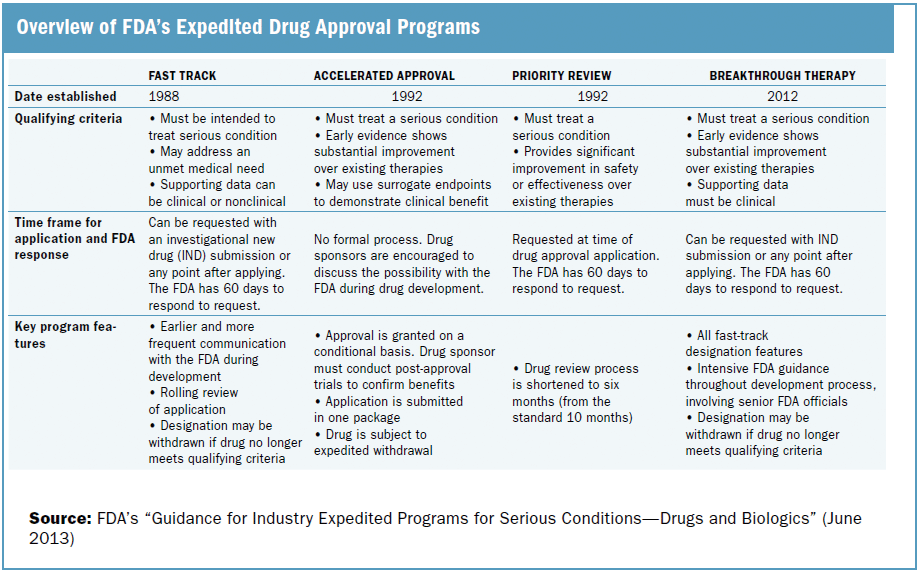
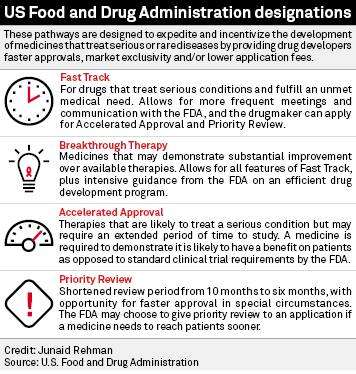
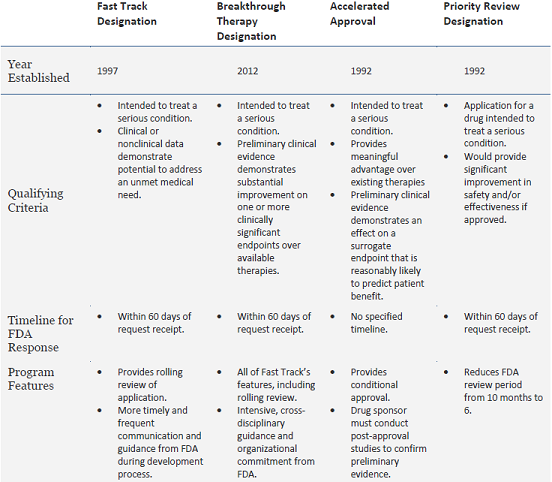
The Need for Speed in Drug Development: A Sponsor's Guide to FDA Expedited Programs | Halloran Consulting Group

Y-mAbs Reports Initiation of Rolling Review of BLA for Naxitamab to the US FDA to Treat Neuroblastoma

Update to Drugs, Devices, and the FDA: How Recent Legislative Changes Have Impacted Approval of New Therapies - ScienceDirect