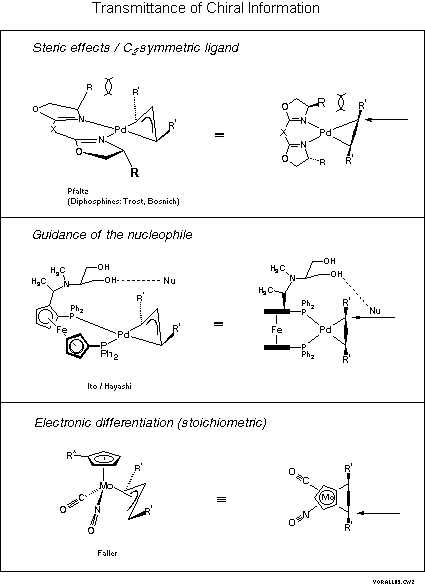
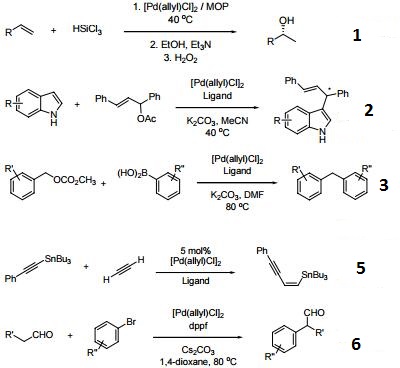
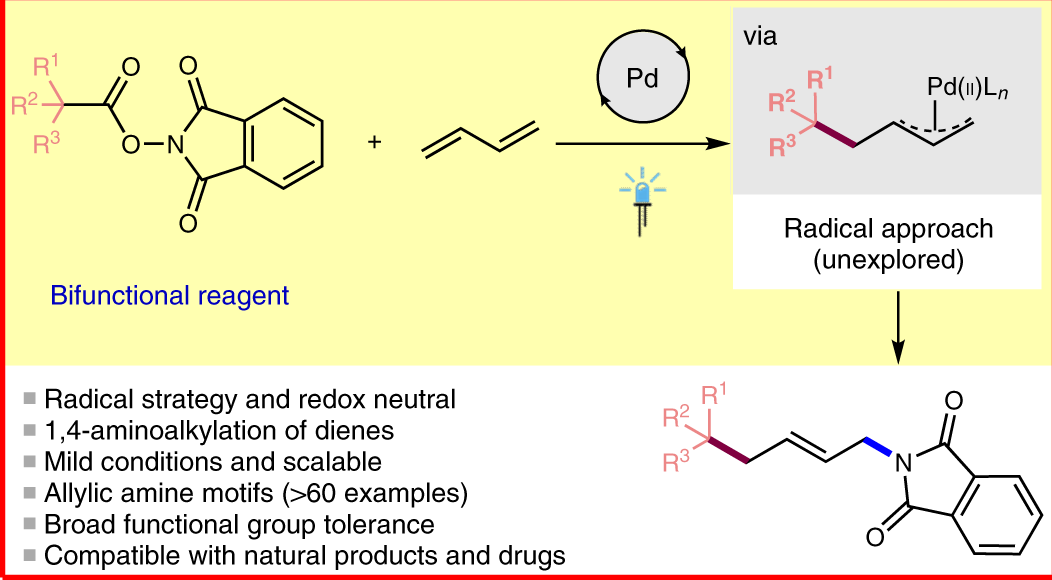
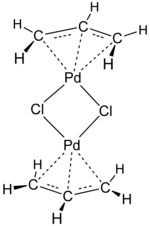
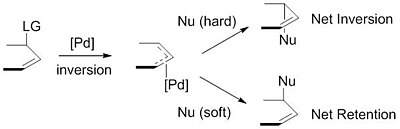
Asymmetric allylic substitution by chiral palladium catalysts: Which is more reactive, major π-allyl Pd(II) species or minor π-allyl species? - ScienceDirect

Buy Reactivity of Cationic (Pi-Allyl)Palladium(ii) Complexes with Olefins and Dienes. Book Online at Low Prices in India | Reactivity of Cationic (Pi- Allyl)Palladium(ii) Complexes with Olefins and Dienes. Reviews & Ratings - Amazon.in

Palladium Enolate Umpolung: Cyclative Diacetoxylation of Alkynyl Cyclohexadienones Promoted by a Pd/SPRIX Catalyst - Takenaka - 2014 - Angewandte Chemie International Edition - Wiley Online Library
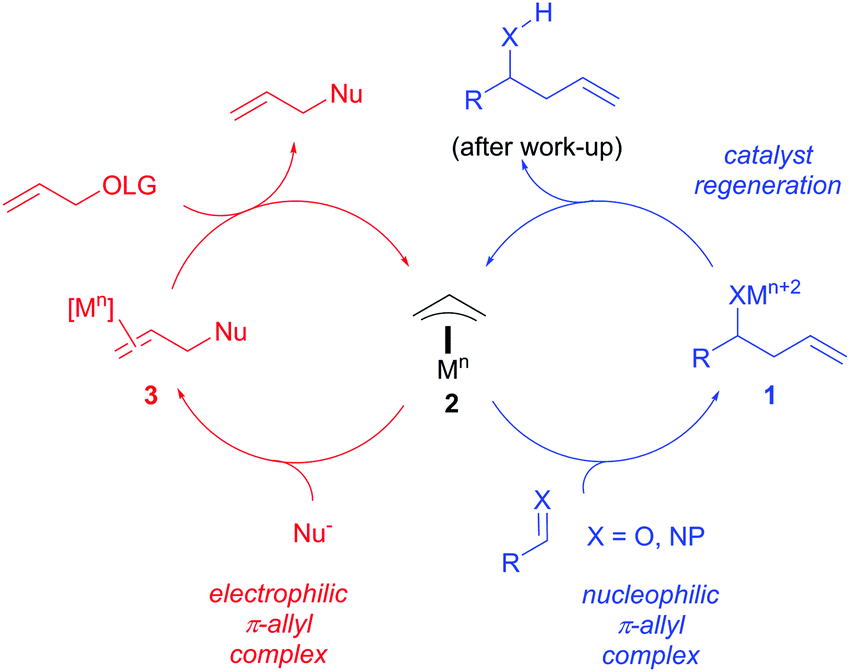
Catalytic nucleophilic 'umpoled' π-allyl reagents - Chemical Society Reviews (RSC Publishing) DOI:10.1039/C7CS00449D

Synthesis and characterization of (π-allyl)palladium(II) complexes containing dialkylbiaryl phosphine ligands - ScienceDirect

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society

π-Allyl)palladium Complexes Bearing Diphosphinidenecyclobutene Ligands (DPCB): Highly Active Catalysts for Direct Conversion of Allylic Alcohols | Journal of the American Chemical Society

Mechanism of allyl deprotection through catalytic palladium π-allyl... | Download Scientific Diagram

Palladium-catalyzed regio- and enantioselective migratory allylic C(sp3)-H functionalization | Nature Communications

Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism | Nature Communications
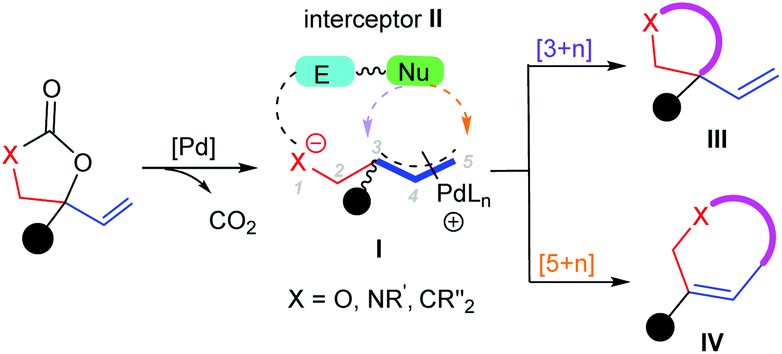
Recent advances in annulation reactions based on zwitterionic π-allyl palladium and propargyl palladium complexes - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D1QO00273B

Palladium‐Catalyzed Electrophilic Allylation Reactions via Bis(allyl) palladium Complexes and Related Intermediates - Szabó - 2004 - Chemistry – A European Journal - Wiley Online Library

Palladium-Catalyzed Asymmetric Allylic Alkylation/α-Iminol Rearrangement: A Facile Access to 2-Spirocyclic-Indoline Derivatives | CCS Chem
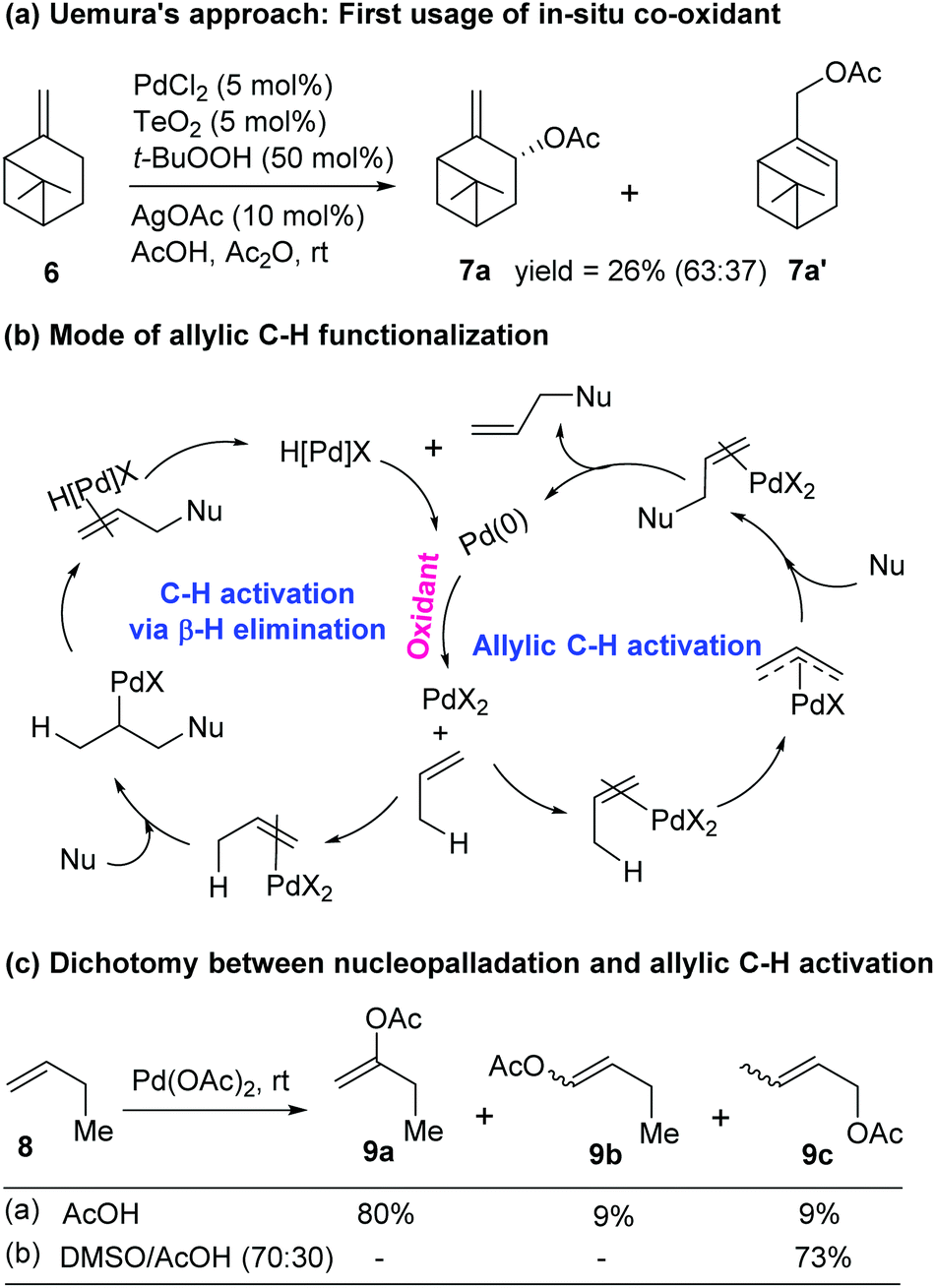
Catalytic allylic functionalization via π-allyl palladium chemistry - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C9OB01725A