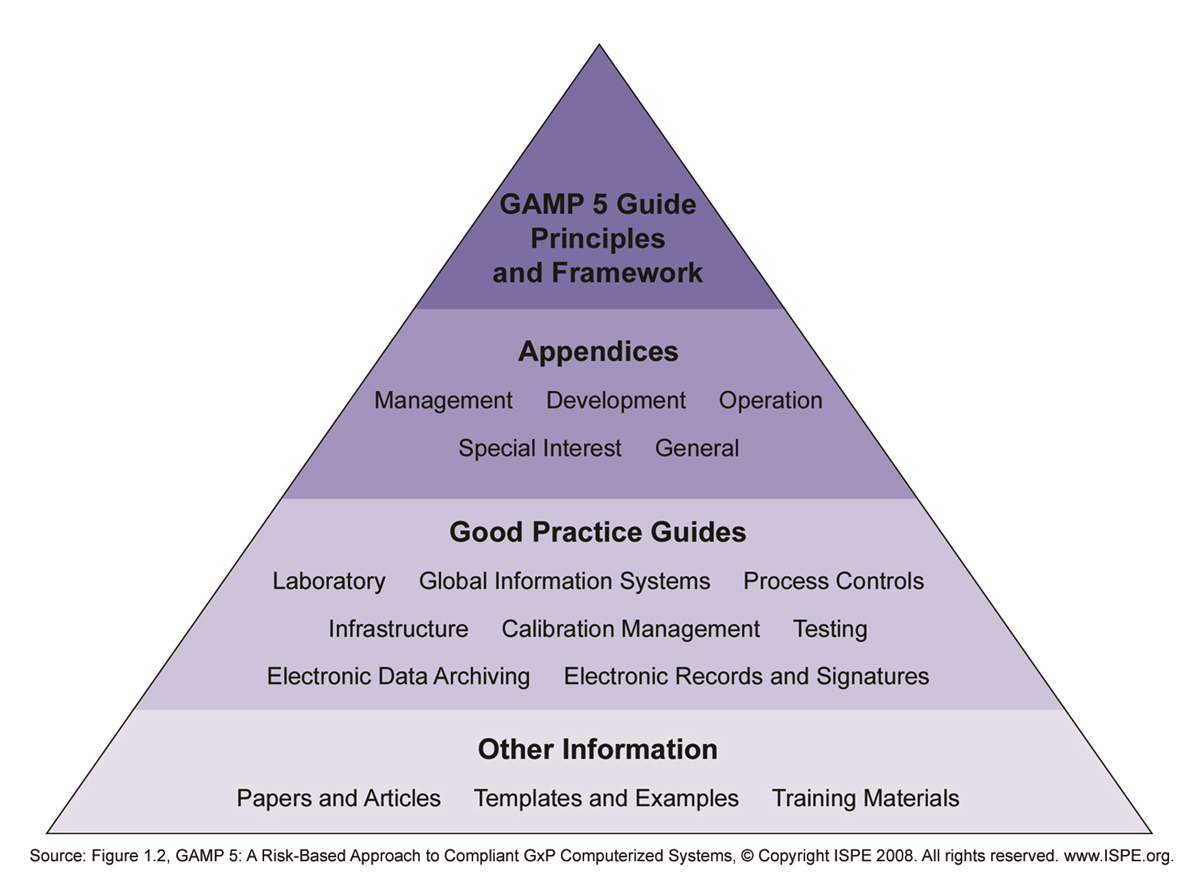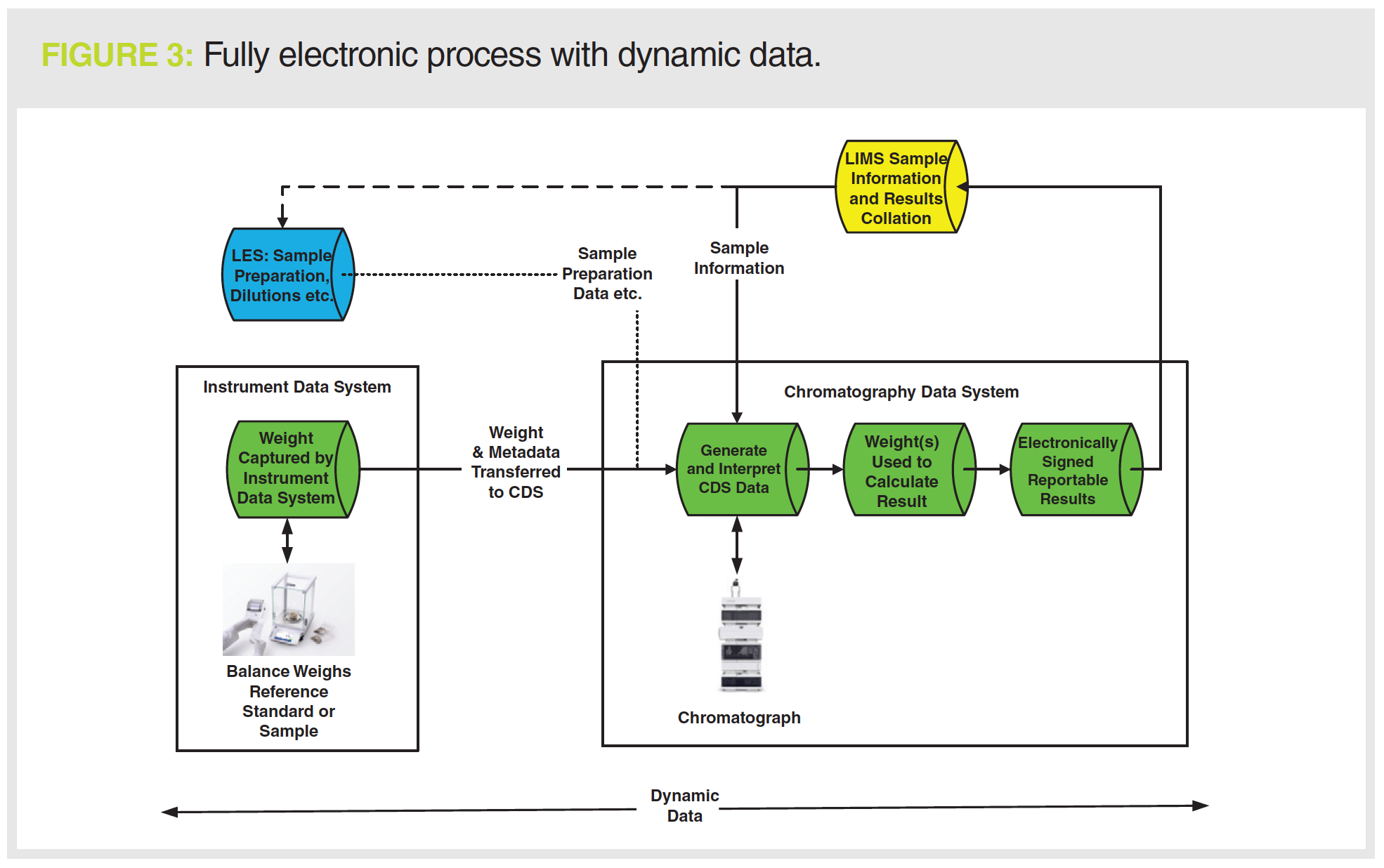
Backup, replication and archiving… What measures to take to preserve the integrity of your data? - A3P - Pharmaceutical & Biotechnology Industry

Gamp Good Practice Guide: GXP Compliant Laboratory Computerized Systems (2 Edition) | PDF | Verification And Validation | Systems Theory